
Principle Green Development Safe Development
Anti-corrosion systems: ISO 12944 standard ranges from C1 to C5.
Release time:
2025-07-07
Corrosion is the phenomenon in which materials—primarily metals—undergo chemical or electrochemical reactions with their surrounding environment, leading to a deterioration of their performance. In the fields of construction, industry, and marine engineering, corrosion prevention has always been a critically important issue. Whether it’s towering steel structures reaching for the clouds or offshore facilities deep in the ocean, corrosion can pose a serious safety hazard.
I. Professional protection, worry-free anti-corrosion
Corrosion is the phenomenon in which materials—primarily metals—undergo chemical or electrochemical reactions with their surrounding environment, leading to a deterioration of their performance. In the fields of construction, industry, and marine engineering, corrosion prevention has always been a critically important issue. Whether it’s towering steel structures reaching into the clouds or offshore facilities deep beneath the sea, corrosion can pose a serious safety hazard.
The international standard ISO 12944 is the most widely used standard for corrosion protection classes and is extensively applied in the protective design of steel structures, industrial equipment enclosures, and outdoor equipment.
II. Classification of Corrosion Resistance Levels
International Standard ISO 12944-2 provides a comprehensive classification and guidance for anti-corrosion coating systems for steel structures. Based on the corrosivity of the environment, atmospheric conditions are categorized into six levels, ranging from C1 (low corrosivity) to CX (extremely high corrosivity):
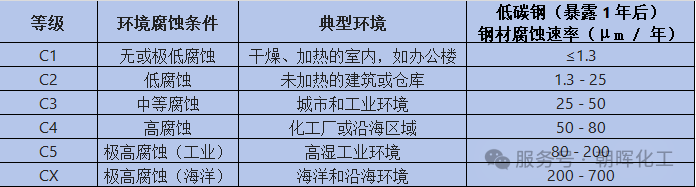
III. Detailed Explanation of Corrosion-Resistant Environments at All Levels
1. C1 Level (Low Corrosivity)
• Typical environment Rural areas or clean urban environments, free from pollution.
Coating Selection Generally, no anti-corrosion coating or even a simple decorative coating is required.


2. C2 Level (Low Corrosivity)
• Typical environment Urban environment, mildly polluted.
Coating Selection :
• Single-layer primer plus a conventional topcoat, such as an epoxy primer plus an acrylic topcoat.


3. C3 Level (Moderate Corrosivity)
• Typical environment Urban industrial environments or coastal areas, moderate pollution.
Coating Selection :
• Three-layer coating system (primer, intermediate coat, topcoat).
• For example: Zinc-rich primer (40–80 μm) + epoxy midcoat (80–160 μm) + polyurethane topcoat (40–80 μm).


4. C4 Level (High Corrosivity)
• Typical environment Industrial environments or coastal areas—high humidity and high pollution.
Coating Selection :
• Epoxy zinc-rich primer (50–80 μm)
• Epoxy mica intermediate coat (100–240 μm)
• Polyurethane topcoat (50–80 μm)
• Total dry film thickness Not less than 200 μm


5. C5 (High Corrosivity)
• Typical environment High-humidity, high-pollution industrial areas, marine environments such as coastal chemical plants, ports, and mining equipment.
Coating Selection :
• Epoxy zinc-rich primer (50–80 μm)
• Epoxy mica intermediate coat (160~360 μm)
• Polyurethane/fluorocarbon topcoat (50–80 μm)
• Total dry film thickness Not less than 260 μm.


6. CX Grade (Extremely Corrosive)
• Typical environment : Extreme corrosive environments in coastal areas, offshore drilling platforms, ship hulls exposed to seawater, or chemically corrosive environments.
Coating system :
• Primer Zinc-rich primer or zinc chromate primer (75–100 μm), providing sacrificial anodic protection.
• Middle coat Epoxy thick-paste coating (180 ~ 400 μm), increasing the thickness of the barrier layer.
• Topcoat Fluorocarbon coatings or polyurethane coatings (50–80 μm) offer resistance to ultraviolet radiation and weathering.



IV. Scientific Principles of Coatings
1. Barrier effect The coating material blocks moisture, oxygen, and corrosive ions.
2. Cathodic protection The zinc-rich coating forms an electrochemical barrier, sacrificing itself to protect the steel.
3. Adhesion The quality of surface treatment directly affects the coating’s service life; sandblasting to meet the Sa 2.5 standard is the optimal choice.
V. Summary
The core of corrosion prevention lies in “scientific matching” and “standardized construction.” On the one hand, it is essential to rationally combine primer, intermediate coat, and topcoat according to different corrosive environments, thereby establishing a layered protective system that specifically resists erosion from salt spray, acids and alkalis, ultraviolet radiation, and other factors. On the other hand, it is crucial to strictly control the construction process—from removing rust and achieving proper surface roughness to managing coating thickness and observing adequate drying intervals between layers—ensuring that every step meets the required standards. Furthermore, professional inspections and subsequent maintenance are indispensable for guaranteeing the long-term effectiveness of the corrosion protection. Only through the synergy of these two aspects can optimal protection be achieved.
Latest news
ZhaoHui Chemical Suzhou Team Building Event
2025-12-09
2025-08-14


























